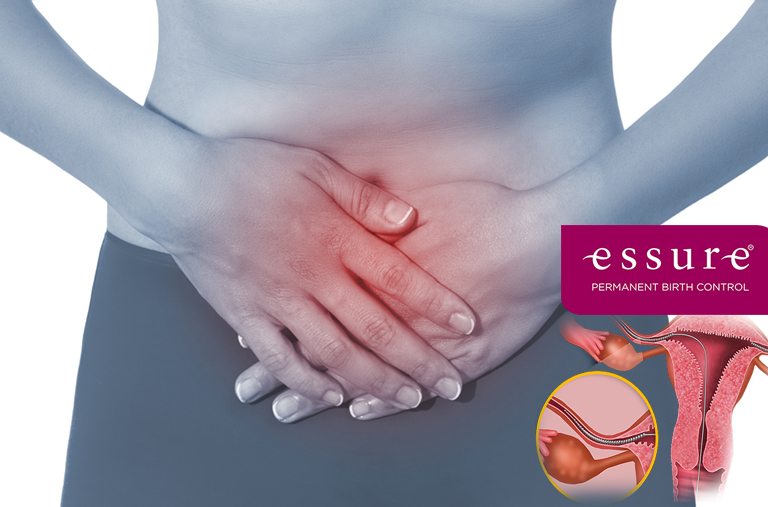
Essure Birth Control Lawsuit
Essure Birth Control Implants was advertised as a safe and permanent birth control but caused long lasting injuries.
Contact our Mass Tort and Class Action attorneys today.
Contact our Mass Tort and Class Action attorneys today.

Essure is a form of permanent, non-hormonal birth control. It was originally manufactured by a company called Conceptus before it was purchased by Bayer. During the non-surgical implantation procedure, two flexible Essure coils made of metal and polyester are inserted through the vagina and uterus and are placed inside each fallopian tube. Tissue begins to develop around the metal coils over the course of a few months, blocking conception. Women who receive the Essure birth control implant must be evaluated by their doctors after three months to ensure that the procedure worked and that they are no longer able to get pregnant. Essure is the only non-surgical method of sterilization for women who want permanent birth control. It has been touted as a safer alternative to tubal ligation. Essure birth control was approved by the U.S. Food and Drug Administration in 2002. It was approved by the FDA though a fast-track review system because it provided an alternative to surgical sterilization and had a much faster recovery time. Now, however, a growing number of women are concerned that the Essure coils were approved before their risks had adequately been investigated.
Hundreds of thousands of women looking for a permanent birth control option have chosen Essure birth control. However, since it was approved, more than 4,500 adverse event reports have been filed with the FDA regarding Essure complications.
Consumer advocate Erin Brockovich has helped to lead a campaign seeking to recall Essure birth control. She, along with thousands across the United States, has complained that Essure coils never should have received approval from the FDA. They are lobbying the federal agency to remove the contraceptive device from the market.
Call an Essure Permanent Birth Control Lawyer Who Will Fight For Your Rights
An Essure lawsuit has been filed against Bayer, accusing the drug manufacturer of misleading women about the permanent birth control. The plaintiff is challenging the premarket approval of Essure, which is currently shielding Bayer from product liability. According to the Essure birth control lawsuit, Essure’s approval was based on questionable studies, and the birth control should not have been put on the market without further study.
Were you prescribed an Essure Birth Control Implant? If you did and you experienced abdominal pain, heavy bleeding, back pain, pelvic pain, pain during intercourse, abdominal bleeding, excessive weight gain, dental problems, excessive rash/hives, or coil removal/hysterectomy you may qualify for compensation.
If the Essure implant separates, breaks and/or moves, attempted removal often requires multiple surgeries, sometimes requiring removal of the uterus entirely.
The U.S. Food and Drug Administration said it will require a new “black box warning” label for this implantable permanent contraceptive device. A black box warning in the labeling of products is “designed to call attention to serious or life-threatening risks,” according to the FDA website. Monday’s announcement comes after more than 5,000 women filed grievances with the FDA between November 2002 and May 2015, complaining of unintended pregnancies, miscarriages, stillbirths and severe pain and bleeding after an implantation.
Reported Essure problems include:
If you or a loved one has experienced complications from Essure Birth Control, contact the office of Chhabra Gibbs & Herrington PLLC for a free consultation. For a free confidential consultation with our Lawyers at our law firm, please call 601-948-8005 or use our live chat.
Contacting the firm is free. We understand that the disputes facing you and your family can seem daunting.