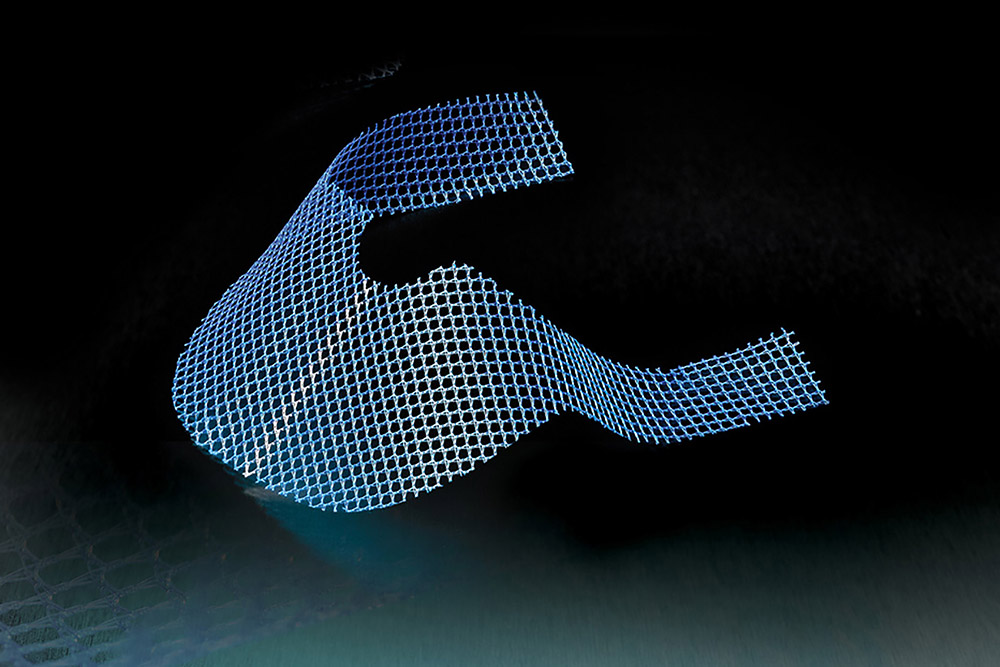
Thousands of women who have received transvaginal mesh implants to treat stress urinary incontinence (SUI) or pelvic organ prolapse (POP) have filed lawsuits in state and federal court to recover compensation for the debilitating injuries they suffered from the transvaginal mesh products.
If you received a transvaginal mesh implant and were injured as a result, you might be eligible for compensation from the mesh’s manufacturer whether or not the particular brand of transvaginal mesh your doctor used has been recalled, or is still on the market.
Transvaginal Mesh Victims Are Recovering Compensation
A victim of injuries from transvaginal mesh manufactured by C.R. Bard recovered $5.5 million in 2012 after her case was tried in California state court, and a victim of injuries resulting from transvaginal mesh manufactured by Johnson & Johnson’s Ethicon unit recovered more than $7 million in 2013 after her case was tried in New Jersey state court.
Most recently, in August 2013, the jury in the first trial of one of the thousands of cases against C.R. Bard pending in West Virginia ordered C.R. Bard to pay $250,000 in compensatory damages and $1.75 million in punitive damages. In that case, Donna Cisson, a public-health nurse from Georgia, allegedly stated that Bard’s Avaulta mesh product, which she was implanted with in 2009, caused her to suffer serious pain and injury, and that she underwent several surgeries to have it removed. Under Georgia law, 75 percent of the $1.75 million punitive-damages award was handed over to the state’s general fund, so, between punitive and compensatory damages, Cisson walked away with about $600,000.
Transvaginal mesh victims have also recovered settlement awards (that is, compensation that resulted from negotiations with the mesh manufacturers lawyers, not from a jury’s decision). In late June 2013, Endo Health Solutions announced that it will pay $55 million to settle an undisclosed number of lawsuits filed by women who claim they suffered serious injuries from transvaginal mesh products manufactured by the company’s American Medical Systems unit (AMS).
Thousands of Transvaginal Mesh Cases Are Still Pending
All federal-level transvaginal mesh lawsuits that are still pending against five mesh manufacturers have been consolidated into five multidistrict litigation (MDL) proceedings in the U.S. District Court for the Southern District of West Virginia. District Court Judge Joseph Goodwin is the presiding judge. The five companies involved are: C.R. Bard; American Medical Systems; Boston Scientific; Ethicon; and Cook Medical.
MDL allows parties whose cases share common opponents, facts, and legal issues to combine their cases for the pretrial phase only. Consolidation saves the parties and the judiciary’s time and resources while allowing plaintiffs the opportunity to recover an amount of compensation that corresponds to their injuries, unlike in a class action lawsuit, which provides a fixed award.
At the end of the pretrial process, the parties select a handful of plaintiffs, whose cases are typical of all plaintiffs, to try their cases before juries in what are called bellwether trials. The first transvaginal mesh bellwether against C.R. Bard was scheduled in July 2013.
There are also lawsuits for transvaginal mesh injuries pending in several state courts around the country.
Chhabra Gibbs & Herrington PLLC Can Help Women Injured by Transvaginal Mesh
The recent jury awards and settlement amounts received by transvaginal mesh victims are proof that perseverance, plus compassionate representation by a qualified attorney, can lead to victory against faulty medical device manufacturers.
If you or someone you know has had surgery for that involved the implantation of surgical mesh, and is experiencing symptoms consistent with malfunction, contact Chhabra Gibbs & Herrington PLLC here by chat or message or calling this number: 601-948-8005.