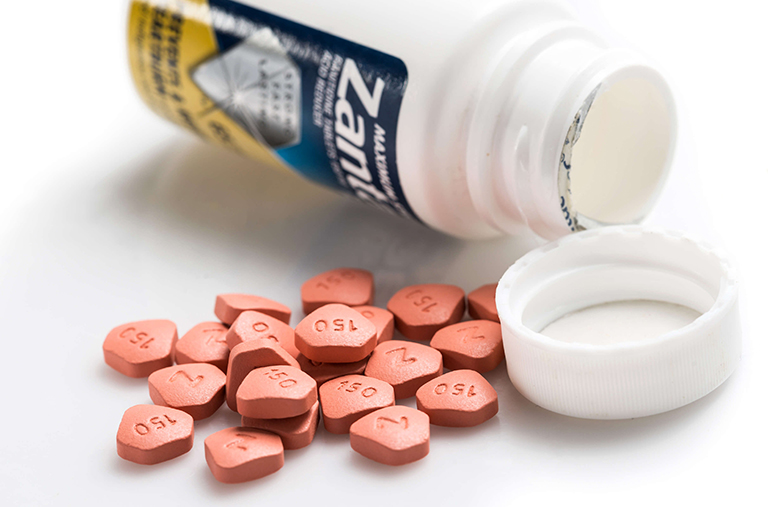
Zantac Lawsuit
The heartburn drug Zantac or its generic form Ranitidine may have been contaminated with NDMA and is linked to cancer.
Contact our Mass Tort and Class Action attorneys today.
Contact our Mass Tort and Class Action attorneys today.

Zantac, or its generic form Ranitidine, is used to treat and prevent the formation of ulcers in the stomach and counteract the accumulation of acid in the stomach that may lead to gastroesophageal reflux disease (GERD).
Recently, the U.S. Food and Drug Administration (FDA) alerted the public that some ranitidine medications, including Zantac, contain low levels of a possible human carcinogen known as N-nitrosodimethylamine (NDMA). The FDA is currently assessing the risk posed to patients from the presence of NDMA. This compound is classified as a potentially dangerous cancer-causing agent, and is used in gasoline, rocket fuel, as a stabilizer in industrial materials and as an additive to lubricants.
Zantac Lawyers are Here for You
Contact our Zantac lawyers to review whether you are a family member may be entitled to financial compensation. If you or a loved one has suffered one or more of these Zantac or Ranitidine complications, you may be entitled to a cash award and compensation for medical expenses. For a free confidential consultation with Zantac Lawyers at our law firm, please call 601-948-8005 or use our live chat.
What is Ranitidine?
Ranitidine belongs to a class of medications known as histamine-2 blockers, which work by lowering the acid levels in the stomach. Zantac is a popular over-the-counter (OTC) medication containing ranitidine used for the treatment of heartburn. Ranitidine is used in both prescription and OTC medications to prevent ulcers and target other conditions associated with the overproduction of stomach acid.
Common brands of ranitidine include:
What is Zantac?
Zantac, generic ranitidine, is a medication that treats stomach acid levels in conditions such as peptic ulcers, gastroesophageal reflux disease (GERD), and Zollinger-Ellison syndrome (excess acid caused by tumors). Research published in 2012 also indicates that it may be effective in treating hives (urticaria). Zantac may be taken orally or injected either into muscle tissue or a vein. Zantac is part of a class of antacids known as histamine (H2) blockers, which prevents the action of the naturally-occuring compound involved in localized immune response (such as sneezing, itching and watery eyes) as well as the regulation of the digestive process.
N-nitrosodimethylamine (NDMA) is a probable carcinogen. It is an industrial waste product, yellowish in appearance with a faint, distinctive odor. Found in cured meats and tobacco smoke, it is a common environmental contaminant. This substance has also been used as a stabilizer in industrial lubricants as well as an ingredient in rocket and jet fuel. In 2018, NDMA contamination was discovered in a number of blood pressure medications known as ARB blockers, such as Valsartan. The source of the contamination was eventually traced back to a factory in China, where changes in the manufacturing process led to a chemical reaction, resulting in the formation of NDMA. It is not yet known how NDMA came to contaminate Zantac.
What are the possible side effects of NDMA-contaminated Zantac?
According to studies of the contaminant NDMA (N-nitrosodimethylamine), possible side effects of ingestion are:
The FDA issued a warning about the presence of NDMA, a probable human carcinogen and known environmental contaminant, in medications containing ranitidine. NDMA is also found in foods, such as cured meats. The FDA had advised numerous recalls of other medications used to treat blood pressure and heart conditions known as Angiotensin II Receptor Blockers based on findings that NDMA was present in these medications. Currently, the FDA is evaluating whether the levels of NDMA in ranitidine warrant a recall of the affected medications. A federal class action lawsuit was filed in California alleging that Zantac’s manufacturer failed to warn users that the drug contains NDMA despite scientific evidence that the carcinogen can be dangerous. The lawsuit alleges that the levels of NDMA in a single Zantac pill far exceeds the acceptable threshold for daily NDMA intake of below 100 nanograms. According to the lawsuit, NDMA is known to cause liver damage, internal bleeding, and possibly death.
If you or a loved one has suffered one or more of these Zantac or Ranitidine complications after taking Zantac for a minimum of 1 year, you may be entitled to a cash award and compensation for medical expenses. For a free confidential consultation with Zantac Lawyers at our law firm, please call 601-948-8005 or use our live chat.
Contacting the firm is free. We understand that the disputes facing you and your family can seem daunting.