Know Your Rights About Covid-19 Related Travel Cancellations
As the Coronavirus continues to spread, many companies, such as airlines, are having Covid-19 related travel cancellations that customers have already purchased tickets for. Some consumers are not being offered a refund. Instead, airlines and other travel companies are forcing consumers to accept vouchers that can only be used with that specific airline and expire within a short period of...

Supreme Court Upholds Right to Stop Political Robo-Calls
On July 6th, the United States Supreme Court upheld the provision of the Telephone Consumer Protection Act (TCPA) that gives citizens the right to stop unwanted robocalls and text messages to their cell phones. The case, which was brought forward by a group of robo-callers, challenged the constitutionality of the TCPA based on the presence of a provision added to the law in 2015 exempting...

Putting A Stop To Robo-Calls
Robo-calls are calls made to consumers using a prerecorded voice message, or mass text. These calls are often made in an attempt to persuade consumers to purchase goods or services from the company behind the calls. Although some robo-calls are made by legitimate companies, including car dealerships, some of these calls may be scams made by individuals or entities in an attempt to...

Zantac Recall
The heartburn and ulcer medication Zantac, also known as Ranitidine, which belongs to the histamine-2 blockers category, has been recalled by the FDA. The FDA has been investigating Zantac for a contaminant known as N-Nitrosodimethylamine (NDMA) in ranitidine medications. The FDA has determined that the impurity increases over time and when stored at higher than room temperatures, that...

Mass Tort Vs. Class Action – What’s The Difference?
We are frequently asked what the difference is between a mass tort and a class action.
What is a Mass Tort?
Unlike a class action, mass torts generally occur when a company causes injury or damage to numerous individuals through a defective product or a singular event such as an explosion at a factory. A company might make a prescription drug or a medical device that causes physical injuries...

What is a Mass Tort?
What is a Mass Tort?
A mass tort is civil litigation involving a large number of victims suing the same corporate defendant(s) over injuries (or even deaths) caused by the defendant’s negligence. In most mass tort cases, plaintiffs are suing over harm caused by a single common product such as a defective drug, medical device or other consumer product.
Mass torts usually begin when numerous...

Class Action Settlement
What is a Class Action Settlement?
A class action settlement is reached when both parties in a class action lawsuit have decided that they no longer want to continue litigating the allegations in the class action lawsuit and want to settle the lawsuit, typically with a monetary benefit to the Class. It’s important to note that a class action settlement is not an admission of guilt on the part...

Talcum Baby Powder Discontinued
On Tuesday, May 19 2020, Johnson & Johnson announced that it was discontinuing all sales of its Talcum baby powder. This announcement includes not only the United States, but Canada as well. This announcement about the talcum powder is a direct effect of the many lawsuits pending against Johnson & Johnson due to cancer.
According to Johnson & Johnson the discontinuing is “due...
Testosterone Product Injuries
The U.S. Food and Drug Administration (FDA) is advising the public that it is evaluating studies showing that prescription testosterone products might cause stroke, heart attacks, and death in men who use them.
What Are Testosterone Products and What Are They Prescribed For?
Testosterone is a hormone that plays a major role in promoting masculine traits and men’s health. The FDA approves...
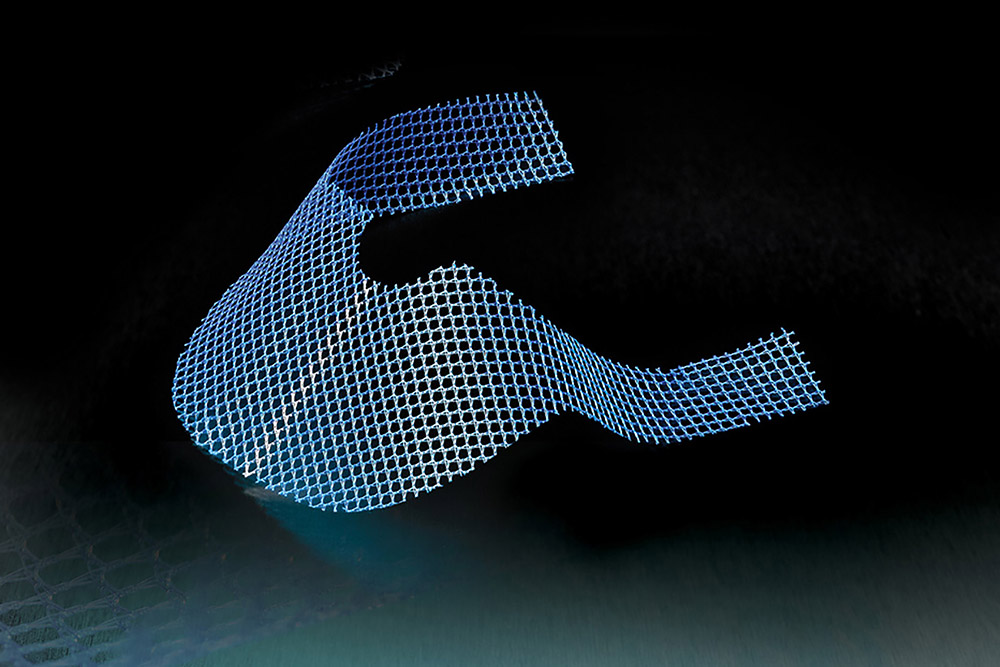
Transvaginal Mesh Pelvic Lawsuits
Thousands of women who have received transvaginal mesh implants to treat stress urinary incontinence (SUI) or pelvic organ prolapse (POP) have filed lawsuits in state and federal court to recover compensation for the debilitating injuries they suffered from the transvaginal mesh products.
If you received a transvaginal mesh implant and were injured as a result, you might be eligible for...